|
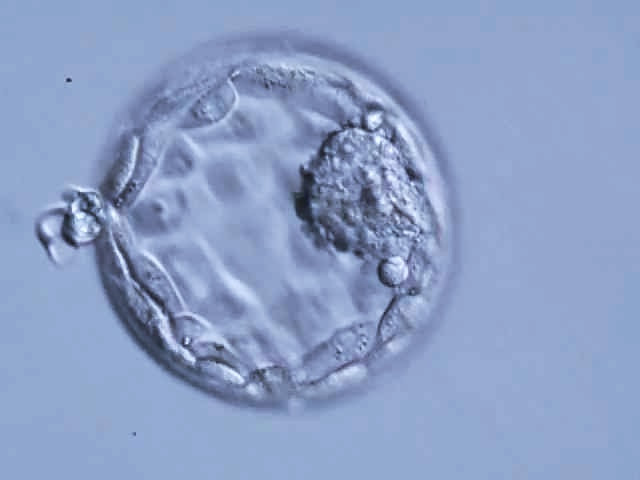
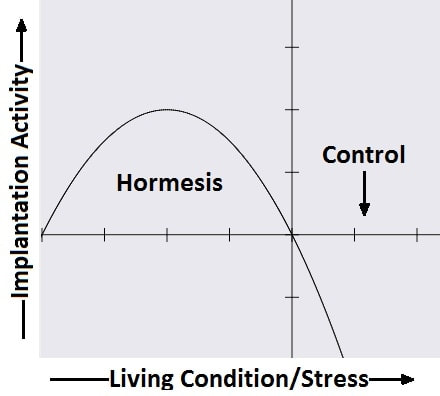
Authors: Javier del Río, Noemi Díaz and Belén Gómez INTRODUCTION What is cryopreservation? The first successful in vitro fertilization (IVF) treatment was in 1978. Since that, there have been a remarkable number of advances in assisted reproductive technologies (ART). Initially, all available embryos were transferred in IVF treatments owing to its low success rate. However, improvements on clinical and laboratory aspects led not only to increased pregnancy rates, but also to increased risk of multiple pregnancies. To prevent this, fewer embryos are transferred and leftover embryos are cryopreserved for potential future cycles use (1). The first pregnancy resulting from transferring a thawed cryopreserved human embryo was reported in 1983 in Australia (2), and the first live birth following embryo cryopreservation was reported in 1984 in The Netherlands (3). Subsequently, the need for an effective cryopreservation program arose from rapid development and improvements of assisted reproductive technology protocols (1). Cryopreservation is a method that requires cells and embryos to be exposed to non-physiological ultra-low temperatures (from -20°C to -196°C) (Fig.2). It aims to achieve “cryogenic suspension of life” through multiple steps, although this puts the elements at risk of damage or “cryoinjury” during temperature changes and phase transitions. These damages could be chilling injury or ice crystal formation, for instance, as a result of the water exchange between the intra- and extracellular compartments, consequence of dramatic changes in osmotic potential (osmotic shock). Therefore, vitrification requires the use of cryoprotectants to avoid the formation of ice crystals in the cells. Two types of cryoprotectants are necessary: permeating and non-permeating. Mixing both at different relative concentrations reduces intracellular ice formation by removing water from inside the cell. Additionally, it creates an osmotic gradient that helps restrict water movement across the cell membrane, thereby preventing osmotic shock (4). There are two typical methods used for cryopreservation: slow freezing and rapid freezing to achieve vitrification. Vitrification is a term used to describe the transformation of a solution into glass by a dramatic increase in viscosity. This method requires to minimize the time for the sample to be exposed to temperature ranges associated with chilling injury and ice crystal formation. As slow freezing, vitrification causes cell dehydration using cryoprotectants. However, unlike that, there is no attempt to maintain equilibrium on both sides of cell membrane (4). The time frame required to reach ultralow temperatures by vitrification is very brief, almost instantaneous. But, the main concern is the need for using high concentrations of cryoprotectant solutions. These might lead to osmotic shock and it could be toxic to cells, affecting embryo survival. Nevertheless, it is possible to limit toxicity by mixing different cryoprotectants, thereby decreasing their relative concentration and the exposure time of embryos to the solution (5). How efficient is the vitrification? This technique seems to be more attractive than slow freezing because it does not require expensive equipment. It uses small amount of liquid nitrogen and it is a simpler technique to perform once the embryologist has gained enough experience in it (6). A recent research performed by Viladimoiv et al. suggests advantages arising from the freezing and thawing process; the authors hypothesize a theory about “cryo-treatment of the embryo”. According to these authors, as a result of freezing or thawing of the embryos there is a decrease in reactive oxygen species levels, in the rate of mitochondrial DNA mutation and cells detoxification is carried out. Also, the authors describe another mechanism involved in restoring the mitochondrial activity (“jumping effect”) which is part of the physiological process of implantation. However, current available data cannot confirm the hypothesis yet (7). Advantages and disadvantages of fresh and frozen cycles Nowadays, fresh embryo transfers (ET) are the most common choice in IVF cycles (8). Nevertheless, in the last years, controlled ovarian stimulation has increased the uncertainty on the possible adverse effects of the ovarian hyperstimulation syndrome (OHSS), and also on possible deleterious effects on the endometrium and implications in obstetric and perinatal results (9). In spite of this, recent developments in cryopreservation of oocytes and embryos have led to substantial improvement in IVF outcomes. This resulted in a significant increase in the number of cycles with frozen embryo transfer (FET), which subsequently led to the enhancement of live births rate (10). What are the advantages of a frozen cycle? Ovarian hyperstimulation syndrome The first strong argument for FET strategy is the prevention of OHSS, that results from an increase in vascular permeability (11,8). OHSS is a medical condition affecting the ovaries of some women who take fertility medication to stimulate oocyte growth. OHSS arguably remains a major cause of morbidity in IVF treatment (10). During a fresh cycle, a woman has to undergo hormonal treatment to regulate her menstrual period, to stimulate the development of multiple oocytes (superovulation), and to encourage their maturation (11, 12). However, in a frozen cycle (FC) the patient does not have to go through ovarian stimulation or egg retrieval depending on their circumstances (13). Many people find that FETs are less stressful than fresh cycles because they do not have to worry about oocytes production or whether there will be viable embryos, since those procedures have already been done (9). Deleterious effects on the embryo The optimization of vitrification protocols has reduced the deleterious effects that this process may produce in embryos. Also, it have been observed similar survival and embryo development in FCs compared to fresh cycles (10). Moreover, best quality embryos, morphologywise, can be stored and transferred in a future cycle in better conditions. These data have allowed for an increment of success rates and the confidence of sanitary personnel and patients over FCs (5). Endometrial receptivity The implantation process, one of the crucial steps in the success of ART, requires a reciprocal interaction between the embryo and the endometrium during a small period of time called window of implantation. This interaction involves the embryo, along with its inherent molecular program of cell growth and differentiation, as well as differentiation of endometrial cells into an adequate uterine receptivity (11). Some patients may find easier to turn to FCs, since dealing with the whole process of medication during a normal cycle for ovarian stimulation may result psychologically and emotionally overwhelming. In this regard, FC may also provide a better outcome (3). The importance of an adequate endometrial environment in ART is highlighted in those patients who resort to oocyte donation, where there must be a synchronization between donor and recipient in fresh cycles. Those cases that require an improvement in endometrial receptivity to stimulate implantation of these donor oocytes seem to obtain better results in frozen cycles or in the next fresh cycle (8). Multiplet pregnancies are one of the major safety concerns of IVF due to the increased risk of neonatal and maternal complications. To achieve good results, to would be ideal to select the optimal single embryo to be transferred. Elective single embryo transfer (eSET) is the most effective way to reduce those risky pregnancies (14). How can cryopreservation damage embryos? Upon analyzing some ART studies and results, embryos are able to adapt and develop in a large range of culture media, showing different gene expression models in different environments. Cryopreservation causes stress in embryos and it is known as “hormesis”(5) (Fig.3). However, if the conditions are too unfavorable or toxic, mitochondrial activity is suppressed below the threshold necessary for the development of the embryo, so that implantation in the endometrium will be affected (5). Results of embryo transfer in fresh cycles vs. frozen cycles The main current objective of IVF professionals is to improve pregnancy rates in both fresh and frozen-thawed cycles. It is clear that embryo and endometrial receptivity are important factors to promote pregnancy rate. Recently, many researches showed FET can enhance the embryo utilization rate and improve the success rate in contrast to other research lines (15). In Roque et al. systematic meta‐analysis for 633 cycles in women aged 27-33 years old showed that FET resulted in a statistically significant increase in the ongoing pregnancy rate and clinical pregnancy compared with the fresh transfer group (8). Interestingly, the fresh group showed a higher miscarriage rate, but no statistical difference was found when compared with the frozen group. According to these data, it seems that the results of IVF-ICSI cycles can be improved by performing the FET especially in patients with normal or high follicular response. This advantage could be explained thanks to a more physiological preparation of endometrium. Several studies have also shown good results with cryopreservation of all embryos and subsequent FET in those patients most susceptible to develop OHSS (8, 16-19). In contrast, Shavit et al. found lower rates of clinical pregnancy and live births in the vitrified-warmed blastocyst group. The difference in implantation and pregnancy rates could be attributed to a higher proportion of good-quality embryos in the fresh blastocysts transfer group. They suggest that in fresh cycles highest quality blastocyst is selected for transfer and the rest are usually vitrified. Thus, vitrified-warmed blastocysts may have slightly poorer grade after warming and prior to transfer (20). In addition, it is necessary to take into account those cycles with frozen oocytes. Braga et al. found that warmed oocytes transferred in endometrial prepared cycles yield better clinical outcomes than fresh ETs. Indeed, they found that fertilization rate, embryo quality, and developmental competence was decreased in embryos derived from vitrified oocytes (12). Conversely, previous studies have suggested that the results of oocyte vitrification followed by ICSI are not inferior with regard to fertilization, embryo developmental competence, pregnancy rates, and live birth (21, 22, 23). An interesting point found in Braga et al. research is that even with lower embryo developmental quality, warmed oocytes transferred in endometrial prepared cycles resulted in higher pregnancy and implantation rates compared with transfer in fresh cycles. This finding strongly suggests that controlled ovarian stimulation impacts endometrial receptivity, which may be a possible cause of implantation failure after ovarian stimulation (12). Indeed, some studies have suggested that pregnancy rate is inversely related to serum progesterone levels on the day of hCG administration (24-27). It has been demonstrated that elevated progesterone levels on hCG trigger day negatively influence the pregnancy, regardless of the oocyte quality. Raised concentrations of progesterone in the late follicular phase are likely to influence the secretory changes of the endometrium, leading to an asynchrony between embryo and endometrial dialogue, which may result in reduced implantation rate (12). Another issue to consider is the obstetric and perinatal outcomes of frozen-thawed cycles. Maheshwari et al. quantified in a meta-analysis the obstetric and perinatal risks for singleton pregnancies after FET and compared it with those after fresh embryo transfer (28). They indicated better perinatal outcomes in singleton pregnancies after the transfer of frozen‐thawed embryos when compared to fresh IVF embryos. This could be explained by antepartum hemorrhage, very preterm birth (delivery at <32 weeks), preterm delivery (delivery at <37 weeks), small for gestational age, low birth weight (birth weight <2500 g), and perinatal mortality significantly lower in women who received frozen embryos than those transferred with fresh embryos (29, 28). It is important to note that most studies comparing perinatal outcome of singleton births conceived after fresh and cryopreserved ETs included both single and multiple ETs. Therefore, part of the adverse perinatal outcome may be attributed to the vanishing twin phenomenon, which occurs in up to 10% of multiple ETs resulting in a singleton live birth (20). What can we conclude? Elective embryo cryopreservation followed by single FET has attracted increasing attention and has been regarded as a potential innovation of IVF treatment. Choosing the well-selected embryo could further increase the chance of live birth of a eSET, which is of high clinical significance. However, there are great gaps in the literature about the risk/benefit ratio of this strategy, which includes multiple steps of treatment (30). The good outcomes in FC might be associated with having a well‐balanced embryo‐endometrium interaction in FC, and also with lacking controlled ovarian hyperstimulation, which may adversely affect endometrial receptivity during fresh IVF cycles. In addition, when hormone replacement cycles were applied in FETs, estrogen and progesterone were given in physiological doses to mimic natural cycles, while supraphysiological doses of gonadotropins were given in fresh cycles (31). On the other hand, other authors find fresh cycles as the best choice, especially in patients who resort to oocyte donation. In fact, it seems that there is a higher proportion of good-quality embryos in fresh blastocysts compared to vitrified-warmed blastocysts, which may have slightly poorer grade after warming and prior to transfer. (8, 20). In conclusion, each case must be individualized in relation to clinical characteristics of the patients and to oocyte, seminal and embryo quality. By doing so, results will be optimized in each cycle and the chances of achieving a live birth will be highly improved. REFERENCES:
1. Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19-26. 2. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305(5936):707-9. 3. Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984; 42(2):293-6. 4. Sparks AE. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin Reprod Med. 2015;33(2):128-44. 5. Konc J, Kanyó K, Kriston R, Somoskői B, Cseh S. Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed Res Int. 2014;2014:307268. 6. Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186-93. 7. Vladimirov IK, Tacheva D, Diez A. Theory about the Embryo Cryo-Treatment. Reprod Med Biol. 2017;16:118–125. 8. Roque M, Lattes K, Serra S, Solá I, Geber S, Carreras R, Checa MA. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156-62. 9. Gurbuz AS, Gode F, Ozcimen N, Isik AZ.Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online 2014;29:541-544. 10. Lattes K, Prat M, Robles A, Carreras R, Brassesco M, Checa MA. Ciclos de criopreservación y vitrificación de ovocitos y embriones: indicaciones y transferencia diferida. Guía 21 de Práctica Clínica de la SEF y de la SEGO. 11. Lessey BA. Endometrial receptivity and the window of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):775-88. 12. Braga D, Setti A, Figueira R, Azevedo M, Iaconelli A, Lo Turco E et al. Freeze-all, oocyte vitrification, or fresh embryo transfer? Lessons from an egg-sharing donation program. Fertil Steril. 2016;106(3):615-622. 13. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3-9. 14. Tobias T, Sharara FI, Franasiak JM, Heiser PW, Pinckney-Clark E. Promoting the use of elective single embryo transfer in clinical practice. Fertil Res Pract. 2016;2(1):1-9. 15. Shen C, Shu D, Zhao X, Gao Y. Comparison of clinical outcomes between fresh embryo transfers and frozen-thawed embryo transfers. Iran J Reprod Med. 2014. Jun;12(6):409–14. 16. Griesinger G, von Otte S, Schroer A, Ludwig AK, Diedrich K, Al-Hasani S, et al. Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof-of-concept study. Hum Reprod. 2007;22(5):1348-1352. 17. D'Angelo A. Ovarian hyperstimulation syndrome prevention strategies: cryopreservation of all embryos. Semin Reprod Med. 2010;28(6):513-518. 18. Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation síndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with ‘‘freeze-all’’ strategy: a prospective multicentric study. Fertil Steril. 2011;95(6):2029-2033. 19. Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593-2597. 20. Shavit T, Oron G, Weon-Young S, Holzer H, Tulandi T. Vitrified-warmed single-embryo transfers may be associated with increased maternal complications compared with fresh single-embryo transfers. Reprod Biomed Online. 2017;35(1):94-102. 21. Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitri- fied donor oocytes in an egg-sharing donation program. Fertil Steril. 2011; 95:1996-2000. 22. Herrero L, Pareja S, Aragones M, Cobo A, Bronet F, Garcia-Velasco JA. Oocyte versus embryo vitrification for delayed embryo transfer: an observational study. Reprod Biomed Online. 2014;29:567-72. 23. Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66-73. 24. Xu, B., Li, Z., Zhang, H., Jin, L., Li, Y., Ai, J. et al, Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97 (1321-7.e1-4). 25. Wu, Z., Li, R., Ma, Y., Deng, B., Zhang, X., Meng, Y. et al, Effect of HCG-day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reprod Biomed Online. 2012;24:511–520. 26. Bosch, E., Labarta, E., Crespo, J., Simon, C., Remohi, J., Jenkins, J. et al, Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–2100. 27. Hamdine, O., Macklon, N.S., Eijkemans, M.J., Laven, J.S., Cohlen, B.J., Verhoeff, A. et al, Elevated early follicular progesterone levels and in vitro fertilization outcomes: a prospective intervention study and meta-analysis. Fertil Steril. 2014;102:448–454.e1. 28. Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368–77.e1. 29. Qiao J, Zhang L, Yan L, Zhi X, Yan J. Female Fertility: Is it Safe to "Freeze?". Chin Med J (Engl). 2015;128(3):390. 30. Wei D, Sun Y, Liu J, Liang X, Zhu Y et al. Live birth after fresh versus frozen single blastocyst transfer (Frefro-blastocyst): study protocol for a randomized controlled trial. Trials 2017; 18(253): 1-7. 31. Zhang L, Yan LY, Zhi X, Yan J, Qiao J. Female Fertility: Is it Safe to “Freeze?” Chin Med J. 2015;128 (3):390-7.
2 Comments
Authors: Inés Abad, Roberto de la Fuente and Sara Sanz INTRODUCTION Fertility treatments are more and more common in our days, reason why it is important to perform these procedures accurately resembling in vivo conditions. Additionally, male factor may oftentimes be underrated, and yet it is 50% of the treatment. The following text aims to establish an updated comparison between in vivo and in vitro semen preparation methods. In the first part a general description of the processes of maturation and capacitation of sperm are presented. MATURATION Where does sperm maturation take place? Once spermatogenesis is completed in the seminiferous epithelium, immature spermatozoa migrate towards the epididymis, the organ in which sperm maturation and storage take place. The epididymis is usually divided into three different parts: caput (head), corpus (body) and cauda (tail) (2). Typical changes in sperm during maturation 1. Acquisition of progressive motility. Even though immature sperm have functional movement machinery, motility of these cells begins in the caput segment. Whereas beating intensity is similar throughout the whole epididymis, flagellar amplitude is modified within this path. This is due to changes on the sperm surface, such as acquisition of new proteins and molecular changes involving inactivation of Ser/Thr phosphatases (3, 4). 2. Migration of the cytoplasmic droplet (CD). This droplet migrates from the neck towards the annulus of the mammalian spermatozoa (in humans, the CD is more proximal, located at the neck as opposed to the distal position of the annulus). The role of this droplet is to regulate ion homeostasis. It contains K+, Cl- and water channels, which have been suggested to work in regulation of sperm volume during the different regions of the epididymis. It also accumulates Ca2+, which has a biphasic role controlling phosphorylation pathways in sperm cells. In immature spermatozoa, it has been hypothesized that high Ca2+ levels found in the CD maintain low levels of tyrosine phosphorylation (5). 3. Changes in sperm protein and lipid profile. - Protein and lipid content Even though changes in these profiles are not well understood, there are three complementary mechanisms that participate in completing maturation:
Perhaps the most important among these changes is the significant reduction in cholesterol content of the sperm membrane. This reduction involves a decrease in the cholesterol/phospholipid ratio that facilitates protein trafficking from and onto the membrane and enhances its fluidity, which will eventually play a role in triggering capacitation and fertilisation (8). - Post-translational protein changes Additionally, certain post-translational modifications of proteins have also been hypothesised to occur during sperm maturation. This is the case for oxidation of thiol groups, which promotes the formation of disulphide bonds (S-S) and stabilises components of both the head and flagellum (9). 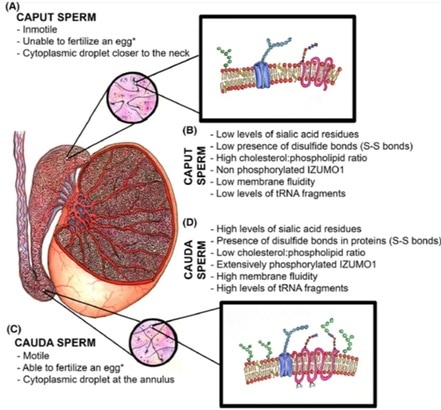 Fig.2. Schematic representation of the main items during sperm maturation. (A) Principal functional and morphological aspects in immature caput spermatozoa. (B) Molecular characteristics of immature spermatozoa. (C) Main morphofunctional traits of cauda mature spermatozoa and (D) their molecular features (4) (*) Ability to fertilize the egg will ultimately depend on completing capacitation. CAPACITATION What is the composition of the ejaculate? In mammals, semen is composed of two different phases:
Following ejaculation, semen is deposited to the anterior wall of the vagina, adjacent to the ectocervical tissues. From here on, for sperm to progress towards the egg through the oviduct or Fallopian tubes, semen must undergo liquefaction. This process usually takes about 20-30 minutes (11). Semen goes through the cervix and reaches the distal portion of the Fallopian tube, where sperm is stored and maintained by interacting with the endosalpingeal epithelium (12, 13). In 1951, Austin and Chang individually observed that a certain period of time in the female tract was required for sperm before fertilization could take place (14, 15). Later, observations in multiple mammalian species confirmed these first notions, and certain studies showed a delay of at least 2 hours before sperm entry into the egg. This supports the hypothesis of sperm maturation before becoming fertile after ejaculation (16). Nowadays, such process is known as capacitation, as opposed to maturation in the male tract explained above. Capacitation and fertility Some of the factors involved in sperm capacitation are steroid hormones such as oestrogens and progesterone, both produced by the follicle. These steroids play different roles: they act as chemoattractants, facilitate triggering of hyperactivation, regulate trafficking of cGMP or modulate the potential for completing acrosome reaction (17-19). Semen liquefaction following ejaculation is mainly modulated by prostate derived peptidase KLK3. In females, KLKs 5–8, 10–11, and 13–15 are expressed at very high levels in the cervix and vagina compared to other adult tissues (20, 21). Moreover, KLK1 and KLK3 transcripts are expressed at the highest level in human endometrium when circulating estradiol (E2) is elevated. These findings suggest that KLKs are expressed in the human reproductive tracts and that some of the KLKs in the uteri are regulated by E2. Abnormal E2 signalling in the female reproductive tract leads to semen liquefaction defects, associated with defective SEMG cleavage and sperm transport, which may result in some cases of infertility. It is known that mice lacking ESR1 (one of the oestrogen receptors) in the epithelial cells are infertile (22), partly due to a reduction in the number of sperm able to reach the oviduct (23). However, the effect of ESR1 loss in the epithelial cells on sperm transport in the uterus has not yet been investigated. Similarly, other potential research lines could investigate liquefaction defects caused by diminished KLK activity in females or regulation of KLKs by molecular signalling in the female tract. Once semen trespasses the cervix, sperm are known to achieve capacitation in an asynchronous fashion during the interaction with the epithelium, which results in a continuous flow of fertile spermatozoa towards the Graafian follicle (24). The ability to bind to the epithelium, in turn, may be indirectly related to the sperm DNA integrity, and so DNA fragmentation levels would be indicative of the fertility potential of the sperm (25). Elements involved in sperm capacitation Even though capacitation had traditionally been regarded as a two-step process, through which changes in the cell membrane would lead to the acrosome reaction (AR) (26), capacitation is currently considered as a continuous process that culminates in the AR. It would be difficult to describe all capacitation-related events separately because all of them are connected to each other in time. However, the most important changes in the sperm during the process are (27-37):
Sperm capacitation is a complex process with multitude of interconnected and highly regulated molecular pathways. One of the first events is the alteration of the permeabilization of the sperm plasma, so that the influx and intracellular concentration of certain ions are increased. The main molecules involved are probably Ca2+ and HCO3-; the net intake by the sperm cell triggers alkalinisation of the pH and the concomitant activation of the soluble adenylyl cyclase (sAC) (38, 39). As an immediate consequence, cAMP levels increase followed by activation of the protein kinase A (PKA) (40, 41). The rise of cAMP causes redistribution of certain phospholipids and proteins of the membrane, and so exposing cholesterol, which accumulates in lipid rafts (42). The organization of these rafts promotes the removal of cholesterol and its translocation to extracellular acceptors like albumin (43). Also, increased cAMP activates PKA, which in turn activates SRC kinase (44). Eventually, SRC kinase activity triggers tyrosine phosphorylation, which results and a wide range of proteins been modified and relocated in capacitating sperm. This has been described in several species, including humans (45). The end result of capacitation is the acrosome reaction (AR), the process by which the content of the acrosome is released to the extracellular environment. In natural conditions, this environment is actually the cummulus cells, whose connections will be broken by the chemical reactions of the acrosomal content, mainly proteases like acrosin and hyaluronidase, also exposed to the right membrane domains during lipid redistribution (46). It is not surprising that mutations affecting any of these processes will result in multiple causes for infertility (47). FUTURE PERSPECTIVES Findings like the one regarding post-ejaculated liquefaction, mutations on acrosome protease-encoding genes or other molecular mechanisms of sperm capacitation are crucial to progress in the field of reproductive medicine, and can lead to: (i) potential diagnostic tools for unexplained infertility cases, (ii) the development of a novel contraception technology to entrap sperm (48), (iii) or even revolutionary new methods for human sperm capacitation in the laboratory (49), which could significantly improve live birth rates for fertility treatments. In the following post... different methods for sperm selection in the laboratory will be explained, paying attention to advantages and disadvantages under different circumstances. The importance of different sperm features like DNA fragmentation or morphology will be discussed in relation to the best sperm selection method to achieve optimal clinical outcomes. REFERENCES:
|
Entries
March 2019
Categories
All
 2016-2019. All Rights Reserved by Embryologist Media. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License . |
Embryologist Media